Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative
quantities of reactants and products in
chemical reactions. In a balanced chemical reaction, the relations among
quantities of reactants and products typically form a ratio of whole numbers.
For example, in a reaction that forms ammonia (NH3), exactly one
molecule of nitrogen (N2) reacts with three molecules of hydrogen (H2)
to produce two molecules of NH3:
N2 + 3H2 → 2NH3
Stoichiometry can be used to find quantities such as the
amount of products (in mass, moles, volume, etc.) that can be produced with
given reactants and percent yield (the percentage of the given reactant
that is made into the product). Stoichiometry calculations can predict how
elements and components diluted in a standard
solution react in
experimental conditions. Stoichiometry is founded on the law of conservation of mass: the mass of
the reactants equals the mass of the products.
Reaction stoichiometry describes the quantitative
relationships among substances as they participate in chemical reactions. In
the example above, reaction stoichiometry describes the 1:3:2 ratio of
molecules of nitrogen, hydrogen, and ammonia.
Composition stoichiometry describes the quantitative (mass)
relationships among elements in compounds. For example, composition
stoichiometry describes the nitrogen to hydrogen ratio in the compound ammonia:
1 mol of ammonia consists of 1 mol of
nitrogen and 3 mol of hydrogen. As the nitrogen atom is about 14 times heavier
than the hydrogen atom, the mass ratio is 14:3, thus 17 kg of ammonia contains
14 kg of nitrogen and 3 kg of hydrogen.
A stoichiometric
amount or stoichiometric ratio of a reagent is the optimum amount or ratio where,
assuming that the reaction proceeds to completion:
1.
All of the reagent is consumed,
2.
There is no shortfall of the reagent,
3.
There is no excess of the reagent.
A non-stoichiometric mixture, where reactions have gone
to completion, will have only the limiting
reagent consumed completely.
While almost all reactions have integer-ratio
stoichiometry in amount of matter units (moles, number of particles), some nonstoichiometric compounds are known that cannot be represented
by a ratio of well-defined natural numbers. These materials therefore violate
the law of definite proportions that forms the basis of stoichiometry
along with the law of multiple proportions.
Gas stoichiometry deals with reactions involving
gases, where the gases are at a known temperature, pressure, and volume, and
can be assumed to be ideal gases.
For gases, the volume ratio is ideally the same by the ideal gas law,
but the mass ratio of a single reaction has to be calculated from the molecular
masses of the
reactants and products. In practice, due to the existence of isotopes, molar masses are used instead when calculating the
mass ratio.
Etymology
The term stoichiometry is derived from the Greek words στοιχεῖον stoicheion "element" and μέτρον metron "measure". In patristic Greek, the word Stoichiometria was used byNicephorus to refer to the number of line counts
of the canonical New Testament and some of the Apocrypha.
Definition
Stoichiometry rests upon the very basic laws that help to
understand it better, i.e., law of conservation of mass, the law of definite proportions (i.e., the law of constant composition) and the law of multiple proportions. In general,
chemical reactions combine in definite ratios of chemicals. Since chemical
reactions can neither create nor destroy matter, nortransmute one element into another, the amount
of each element must be the same throughout the overall reaction. For example,
the amount of element X on the reactant side must equal the amount of element X
on the product side.
Chemical reactions, as macroscopic unit operations,
consist of simply a very large number of elementary reactions, where a single molecule
reacts with another molecule. As the reacting molecules (or moieties) consist
of a definite set of atoms in an integer ratio, the ratio between reactants in
a complete reaction is also in integer ratio. A reaction may consume more than
one molecule, and the stoichiometric
number counts this number,
defined as positive for products (added) and negative for reactants (removed).[1]
Different elements have a different atomic mass,
and as collections of single atoms, molecules have a definite molar mass,
measured with the unit mole (6.02 × 1023 individual molecules, Avogadro's constant). By definition, carbon-12
has a molar mass of 12 g/mol. Thus to calculate the stoichiometry by mass, the
number of molecules required for each reactant is expressed in moles and
multiplied by the molar mass of each to give the mass of each reactant per mole
of reaction. The mass ratios can be calculated by dividing each by the total in
the whole reaction.
Balancing chemical reactions
Stoichiometry is often used to balance chemical equations
(reaction stoichiometry). For example, the two diatomic gases, hydrogen and oxygen, can
combine to form a liquid, water, in an exothermic reaction, as described by the
following equation:
2H2 + O2 → 2H2O
Reaction stoichiometry describes the 2:1:2 ratio of
hydrogen, oxygen, and water molecules in the above equation.
The term stoichiometry is also often used for the molar proportions of elements in
stoichiometric compounds (composition stoichiometry). For example, the
stoichiometry of hydrogen and oxygen in H2O is 2:1. In
stoichiometric compounds, the molar proportions are whole numbers.
Stoichiometry is not only used to balance chemical
equations but also used in conversions, i.e., converting from grams to moles,
or from grams to millilitres. For example, to find the number of moles in 2.00
g of NaCl, one would do the following:
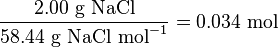
In the above example, when written out in fraction form,
the units of grams form a multiplicative identity, which is equivalent to one
(g/g=1), with the resulting amount of moles (the unit that was needed), is
shown in the following equation,

Stoichiometry is also used to find the right amount of reactants to use in a chemical
reaction (stoichiometric
amounts). An example is shown below using the thermite
reaction,

This equation shows that 1 mole of aluminium oxide
and 2 moles of iron will be produced with 1 mole of iron(III)
oxide and
2 moles of aluminium. So, to completely react with 85.0 g of iron(III) oxide (0.532 mol), 28.7 g
(1.06 mol) of aluminium are needed.


Different stoichiometries in competing reactions
Often, more than one reaction is possible given the same
starting materials. The reactions may differ in their stoichiometry. For
example, the methylation of benzene (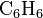 ),
through a Friedel-Crafts reaction using
),
through a Friedel-Crafts reaction using  as
catalyst, may produce singly methylated
as
catalyst, may produce singly methylated  ,
doubly methylated
,
doubly methylated 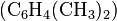 , or still more highly methylated
, or still more highly methylated  products, as shown in the following
example,
products, as shown in the following
example,
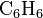 ),
through a Friedel-Crafts reaction using
),
through a Friedel-Crafts reaction using  as
catalyst, may produce singly methylated
as
catalyst, may produce singly methylated  ,
doubly methylated
,
doubly methylated 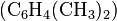 , or still more highly methylated
, or still more highly methylated  products, as shown in the following
example,
products, as shown in the following
example,


In this example, which reaction takes place is controlled
in part by the relative concentrations of the reactants.
Stoichiometric coefficient
In layman's terms, the stoichiometric
coefficient (or stoichiometric number in the IUPAC nomenclature[2])
of any given component is the number of molecules which participate in the
reaction as written.
For example, in the reaction CH4 + 2 O2 → CO2 + 2 H2O, the stoichiometric coefficient of CH4 would be 1 and the stoichiometric coefficient of O2 would be 2.
For example, in the reaction CH4 + 2 O2 → CO2 + 2 H2O, the stoichiometric coefficient of CH4 would be 1 and the stoichiometric coefficient of O2 would be 2.
In more technically-precise terms, the stoichiometric
coefficient in a chemical
reaction system of the i–th component is defined as

or

where Ni is the number of molecules of i,
and ξ is the progress variable or extent
of reaction (Prigogine &
Defay, p. 18; Prigogine, pp. 4–7; Guggenheim,
p. 37 & 62).
The extent
of reaction ξ can be regarded
as a real (or hypothetical) product, one molecule of which is produced each
time the reaction event occurs. It is the extensive quantity describing the
progress of a chemical reaction equal to the number of chemical
transformations, as indicated by the reaction equation on a molecular scale,
divided by the Avogadro constant (it is essentially the amount of chemical
transformations). The change in the extent of reaction is given by dξ = dnB/νB,
where νB is the stoichiometric number of any
reaction entity B (reactant or product) an dnB is the corresponding amount.[3]
The stoichiometric coefficient νi represents the degree to which a
chemical species participates in a reaction. The convention is to assign
negative coefficients to reactants (which are consumed) and positive ones
to products. However, any
reaction may be viewed as "going" in the reverse direction, and all
the coefficients then change sign (as does the free energy). Whether a reaction actually will go in the arbitrarily-selected forward
direction or not depends on the amounts of the substances present
at any given time, which determines the kinetics and thermodynamics, i.e., whether equilibrium lies to the right or the left.
If one contemplates actual reaction mechanisms, stoichiometric
coefficients will always be integers,
since elementary reactions always involve whole molecules. If one uses a
composite representation of an "overall" reaction, some may be rational fractions. There are often chemical
species present that do not participate in a reaction; their stoichiometric
coefficients are therefore zero. Any chemical species that is regenerated, such
as a catalyst,
also has a stoichiometric coefficient of zero.
The simplest possible case is an isomerism

in which νB = 1 since one molecule of B is produced each time the reaction
occurs, while νA =
−1 since one molecule of A is necessarily consumed. In any
chemical reaction, not only is the total mass conserved but also the numbers of atoms of each kind are conserved, and this imposes
corresponding constraints on possible values for the stoichiometric
coefficients.
There are usually multiple reactions proceeding
simultaneously in any natural reaction system, including those in biology.
Since any chemical component can participate in several reactions
simultaneously, the stoichiometric coefficient of the i–th component in the k–th reaction is defined as

so that the total (differential) change in the amount of
the i–th component is

Extents of reaction provide the clearest and most
explicit way of representing compositional change, although they are not yet
widely used.
With complex reaction systems, it is often useful to
consider both the representation of a reaction system in terms of the amounts
of the chemicals present { Ni } (state variables), and the representation
in terms of the actual compositional degrees of freedom, as
expressed by the extents of reaction { ξk }. The
transformation from a vector expressing the extents to a vector
expressing the amounts uses a rectangular matrix whose
elements are the stoichiometric coefficients [ νi k ].
The maximum and
minimum for any ξk occur whenever the first of the
reactants is depleted for the forward reaction; or the first of the
"products" is depleted if the reaction as viewed as being pushed in
the reverse direction. This is a purely kinematic restriction on the reaction simplex,
a hyperplane in composition space, or N‑space, whose dimensionality equals the number of linearly-independent chemical reactions. This is
necessarily less than the number of chemical components, since each reaction
manifests a relation between at least two chemicals. The accessible region of
the hyperplane depends on the amounts of each chemical species actually
present, a contingent fact. Different such amounts can even generate different
hyperplanes, all of which share the same algebraic stoichiometry.
In accord with the principles of chemical
kinetics and thermodynamic equilibrium, every chemical
reaction is reversible, at
least to some degree, so that each equilibrium point must be an interior point of the simplex. As a consequence,
extrema for the ξ's will not occur unless an experimental system is prepared
with zero initial amounts of some products.
The number of physically-independent
reactions can be even greater than the number of chemical components, and
depends on the various reaction mechanisms. For example, there may be two (or
more) reaction paths for the isomerism above. The reaction
may occur by itself, but faster and with different intermediates, in the
presence of a catalyst.
The (dimensionless) "units" may be taken to be molecules or moles.
Moles are most commonly used, but it is more suggestive to picture incremental
chemical reactions in terms of molecules. The N's
and ξ's are reduced to molar units by dividing by Avogadro's number. While dimensional mass units may be used, the comments about
integers are then no longer applicable.
Stoichiometry matrix
In complex reactions, stoichiometries are often represented in a more compact form called the stoichiometry matrix. The stoichiometry matrix is denoted by the symbol,  .
.
 .
.
If a reaction network has 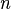 reactions and
reactions and  participating molecular species then the stoichiometry matrix will have corresponding
participating molecular species then the stoichiometry matrix will have corresponding  rows and
rows and 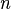 columns.
columns.
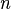 reactions and
reactions and  participating molecular species then the stoichiometry matrix will have corresponding
participating molecular species then the stoichiometry matrix will have corresponding  rows and
rows and 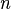 columns.
columns.
For example, consider the system of reactions shown
below:
S1 → S2
5S3 + S2 → 4S3 + 2S2
S3 → S4
S4 → S5.
This systems comprises four reactions and five different
molecular species. The stoichiometry matrix for this system can be written as:

where the rows correspond to S1, S2,
S3, S4 and
S5, respectively. Note that the process of converting a reaction
scheme into a stoichiometry matrix can be a lossy transformation, for example,
the stoichiometries in the second reaction simplify when included in the
matrix. This means that it is not always possible to recover the original
reaction scheme from a stoichiometry matrix.
Often the stoichiometry matrix is combined with the rate
vector, v to form a compact equation describing the rates of change of the
molecular species:

Gas stoichiometry
Gas stoichiometry is the quantitative relationship
(ratio) between reactants and products in a chemical
reaction with
reactions that produce gases. Gas stoichiometry
applies when the gases produced are assumed to be ideal,
and the temperature, pressure, and volume of the gases are all known. The ideal
gas law is used for these calculations. Often, but not always, the standard temperature and pressure (STP) are taken as 0 °C and 1 bar
and used as the conditions for gas stoichiometric calculations.
Gas stoichiometry calculations solve for the unknown volume or mass of a gaseous product or reactant. For
example, if we wanted to calculate the volume of gaseous NO2produced
from the combustion of 100 g of NH3, by the reaction:
4NH3 (g) + 7O2 (g) → 4NO2 (g) + 6H2O (l)
we would carry out the following calculations:
There is a 1:1 molar ratio of NH3 to NO2 in the above balanced combustion
reaction, so 5.871 mol of NO2 will
be formed. We will employ the ideal gas law to solve for the volume at 0 °C
(273.15 K) and 1 atmosphere using the gas law
constant of R =
0.08206 L · atm · K−1 ·
mol−1 :
Gas stoichiometry often involves having to know the molar mass of a gas, given the density of that gas. The ideal gas law can be
re-arranged to obtain a relation between thedensity and the molar mass of an ideal gas:
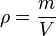 and
and 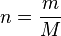
and thus:
| where: | |
 | = absolute gas pressure |
|---|---|
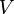 | = gas volume |
 | = number of moles |
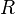 | = universal ideal gas law constant |
 | = absolute gas temperature |
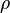 | = gas density at  and and  |
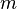 | = mass of gas |
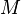 | = molar mass of gas |
Stoichiometry of combustion
In the combustion reaction, oxygen reacts with the fuel,
and the point where exactly all oxygen is consumed and all fuel burned is
defined as the stoichiometric point. With more oxygen (overstoichiometric
combustion), some of it stays unreacted. Likewise, if the combustion is incomplete
due to lack of sufficient oxygen, fuel remains unreacted. (Unreacted fuel may
also remain because of slow combustion or insufficient mixing of fuel and
oxygen - this is not due to stoichiometry.) Different hydrocarbon fuels have a
different contents of carbon, hydrogen and other elements, thus their
stoichiometry varies.
Fuel
|
Percent fuel by mass
|
||
Gasoline
|
14.7 : 1
|
—
|
6.8%
|
Natural gas
|
17.2 : 1
|
9.7 : 1
|
5.8%
|
Propane (LP)
|
15.67 : 1
|
23.9 : 1
|
6.45%
|
Ethanol
|
9 : 1
|
—
|
11.1%
|
Methanol
|
6.47 : 1
|
—
|
15.6%
|
Hydrogen
|
34.3 : 1
|
2.39 : 1
|
2.9%
|
Diesel
|
14.5 : 1
|
0.094 : 1
|
6.8%
|
Gasoline engines can run at stoichiometric air-to-fuel
ratio, because gasoline is quite volatile and is mixed (sprayed or carburetted)
with the air prior to ignition. Diesel engines, in contrast, run lean, with
more air available than simple stoichiometry would require. Diesel fuel is less
volatile and is effectively burned as it is injected, leaving less time for
evaporation and mixing. Thus, it would form soot (black smoke) at
stoichiometric ratio.

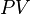
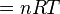
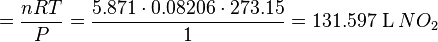

why diesel fuel is less stable and effective if burned, leaving little time for evaporation and mixing that will form soot (black smoke) in the stoichiometric ratio?
BalasHapusbecause diesel fuel has an octane number is so low that, not as good as the combustion of gasoline has a very high octane number and that is causing a black smoke (soot) are dense and numerous.
BalasHapus